|
A new technique
has been added to bariatric surgery: the Implantable Gastric Stimulation
(IGS), which seems to be able to induce satiety while avoiding the
drawbacks of malabsorptive or restrictive usual techniques (refer
to chapters dealing with obesity surgery or bariatric surgery).
These procedures usually entail a substantial modification of the
upper gastro-intestinal tract, regarding both its anatomy and its
function.
An
experimental protocol was initiated by the US company Transneuronix,
on the basis of Dr Valerio Cigaina’s works (an Italian surgeon),
consisting of a randomized clinical study launched in Europe (Austria,
Sweden, Italy, Germany, and France) and in the States at the beginning
of year 2000. After approval by the ethics committee, the French
centre of experimentation was located in Lyon. The device consists
of a stimulation lead implanted in the gastric wall and connected
to an electric programming unit implanted subcutaneously:
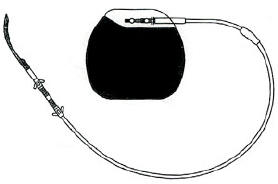
It is very similar to the pacemakers used in cardiology, and can
be implanted by laparoscopy: the Transcend IGS system is made up
of three parts, the Implantable Gastric Stimulator (IGS), the lead
and the programmer. The first is the IGS which is a small metal
box with electronic circuitry. It looks like a heart pacemaker and
is the size of a pocket watch. Its purpose is to generate electronic
pulses. It is implanted under the skin just below the rib cage on
the front of the abdomen. The second part of the system is the lead.
It is a thin, insulated electrical wire, which carries the electronic
signals from the IGS to the stomach. The third part is the programmer.
It is a computer that is connected to a hand held wand. It is like
a remote control that can check how the IGS is functioning and change
the type of electrical signals.
It is very similar to the pacemakers used in cardiology, and can
be implanted by laparoscopy: the Transcend IGS system is made up
of three parts, the Implantable Gastric Stimulator (IGS), the lead
and the programmer.
The IGS is a battery operated device that should require replacement
every 2-5 years. It is not possible to determine exactly how long
your battery will last because the longevity of the battery depends
on many factors. The procedure to replace the IGS is considered
minor surgery and usually requires local anesthesia.
There are certain risks associated with this as well as any other
operation, as well as complications that may occur as a result of
having any foreign object implanted.
Patients
have been enrolled according medical guidelines :
Inclusion criteria :
- Age : 18-50 years.
-
BMI of 40 to 55.
- Documented history of five years of obesity with a failure to
achieve and maintain weight loss with non surgical weight control.
- Patients able and willing to fulfill study requirements (strict
follow-up, informed consent …)
Exclusion
criteria :
- Pregnant or lactating patient.
- Prior surgery of Gastro-intestinal tract as therapy for morbid
obesity.
- Prior surgery on the stomach for any reason.
- Other implantable electrostimulation device (pacemaker).
- Patient at high risk of developing gastric ulcer.
- Patient with motility disorders of the GI tract.
- Patient with a history of cardiac arrhythmia or severe cardiac
disease.
- Patient with poorly or uncontrolled diabetes.
- Patient with any serious health condition not related to their
weight.
Concerning
cautions to be observed, they can be summarized as:
How the IGS is affected by other devices and medical therapies :
Most devices that you will encounter in your daily life - such as
properly operating microwave ovens, cell phones … will not affect
your IGS. However, strong magnets, hair clippers, vibrators, loudspeakers
and similar electrical devices that have a strong static or pulsing
magnetic field can cause your IGS to begin delivering stimulating
pulses. Keep such devices at least 15 centimeters (six inches) away
from where your device is implanted.
Some metal detectors (with gateways for people to walk through)
found in airports, public libraries, etc. may briefly cause an increase
in your stimulation level as you pass through them. Show the authorized
personnel your ID card and point out the location of your device
so that it can be checked with a hand-held monitor.
Your device can be adversely affected by some medical therapies
such as defibrillation, electrocautery, lithotripsy, magnetic resonance
imaging and therapeutic radiation. Make sure that all medical personnel
who treat you are aware that you have a gastric stimulator implanted.
The stimulating pulses emitted by your IGS will be able to be picked
up on a routine electrocardiogram. Tell your doctor that you have
had a gastric stimulator implanted.
Comments:
Comprehensive data will not be available by the end of the year
2001, because of the protocol approved by the American Food and
Drug Administration. However, early results authorize us to do two
observations:
- It is a technically simple and reproducible procedure, which can
be improved furthermore.
- No conclusion regarding the efficacy of the IGS for weight loss
can be made so far.
The
gastric stimulation was object of many others studies as a base
to treat other rare pathologies: Gastro paresis and others stomach
motor disorders. These syndromes are expressed by vomits, dehydratation,
and metabolic and nutritional complications, affecting mainly the
diabetes patients. It is a paradox to propose the same therapy for
one problem completely inverse (the big obese patients), but the
stimulation parameters are much different in this latter case, particularly
the amplitude of applied current and mainly the intervals between
the stimulations, much more frequent for obesity. In the first case,
the intention is to produce contractions of the gastric muscle to
improve the evacuation of a meal. In the second, the amplitude and
the rate of the applied current should lie above the threshold of
both muscle and nerve. Which is more important is currently unknown
and will be determined in future investigations. It is expected
also an effect of the neuro hormonals mediators.
The absence of food restriction is probable the major benefit of
the IGS, also the absence of secondary effects connected to the
malabsorption procedures. One could question about the permanence
of the satiety while the patient is stimulated and about the patient
capacity to overcome this in a long term basis. It is anticipated
that a dietary and a psychological reeducation will play a more
important role in the eventual success of the IGS than in the others
bariatric procedures.
The IGS is a potential alternative to the others surgical methods
to treat the morbid obesity. In 2001, the specific indications for
these different techniques are not precise, and the choosing of
a specific procedure remains in the great majority connected to
the experience of each surgeon, which varies from one country to
another. The future of the IGS faces certainly the comparison against
the classical procedures.
|